Crystallization is a physical change. Crystallization is the formation of solids from the liquid or gaseous phase. This technique includes obtaining the crystals of a soluble substance from a hot saturated solution and separating the soluble solid from the solution.
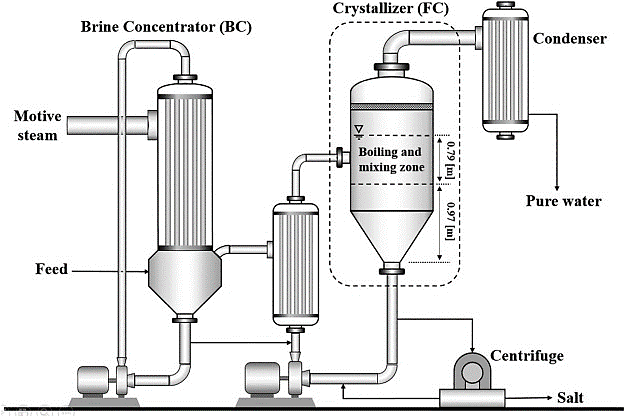
To concentrate feed into solid crystals and clean water, crystallizers are used. A progressively harder method for which crystallite sizes are formed from a liquid solution is known as crystallization. Crystallizers can remove liquid wastes completely, resulting in no liquid discharge (ZLD). The process continues and secondary crystallization are the two stages of crystallization. The formation of new crystals is referred to as primary nucleation. Secondary nucleation is the primary stage that results in the mass production of crystals. There are two types of crystallization processes: evaporative crystallization and cooling crystallization.
The crystallization course of action is hard and The key move in the development of good crystals inside of a liquid solution, the solution is concentrated and cooled until the solute focus is better than dissolved at that temperature along with the solute forms pure crystals.
The ChilledCrys membrane crystallizer system will not be a silver bullet that is certainly ideal for all brines. Having said that, for certain saltwater brines present in sector (e.
The higher drinking water evaporation charge of forty eight.0 kg m−2 each day reached from the solar crystallizer array in outside discipline test also suggests its fantastic probable for simple brine therapy. More enhancements from the overall performance on the solar crystallizer might be multi-faceted, like although not restricted to making use of artificial wind discipline to boost environmental thermal Power harvesting, optimizing array assembly. The solar crystallizer is thought broadly applicable to numerous crystallization processes, like salt recovery from squander brines, salt mineral extraction from salt lake, and thus is really a significant contribution to your demanding difficulty of industrial brine cure with ZLD.
a SEM impression in the crystals soon after absolutely drying concentrated RO waste brine straight on wafer and also the corresponding EDS maps of b metallic things, c magnesium, d chloride, e sulfur, and f oxygen.
The supersaturation filling method can be reached by cooling the solution but with negligible or slight evaporation.
A lab-scale setup was designed To judge the photo voltaic evaporation and crystallization performance on the solar crystallizer. A solar simulator (Oriel solar simulator) was utilized to supply solar radiation with a constant depth of a thousand W m−two. A mass equilibrium (ME204E, Mettler Toledo) was utilized to history the h2o mass alter. The temperature distribution was monitored by an IR camera (A600-collection, FLIR). The evaporation fees on the solar crystallizer with distinct heights when treating pure water have been measured less than a person Solar illumination for three h.
The h2o is often recycled again into the plant processes, while the solids that are uncomplicated to handle is often securely disposed of in an authorised squander disposal facility.
After the salt crust layer was directly taken off by a plastic spatula with no water washing treatment method, the 3D photo voltaic crystallizer was able to deliver a similar evaporation overall performance in the next 24-h exam cycle (Fig. 4a), indicating that the 3D solar crystallizer is often effortlessly regenerated and reused without the need of recognizable change in true brine treatment effectiveness. It had been recognized that, if the solar light was turned off during the Procedure, the evaporation charge dropped to around 0.three kg m−two h−one (Fig. 4a). Even so, the surface area salt layer didn't exhibit any apparent change even once the solar crystallizer was saved in darkish for 12 h (Fig.
The competitive benefit of the outer floor in salt precipitation keeps the interior facet with the membrane free of salt crystals (Fig. 3d) and maintains the water channel In the QGF membrane unobstructed.
In this kind, the crystals are fashioned from the answer when it reacts. This crystallization system is faster than other kinds of crystallization as the response method is rapid. The speedy conversion period signifies the shortest home time in all precipitation procedures.
There is generally a side opening to aid entry to the manual extraction Crystallizer Manufacturer Crystallizer For Zero Liquid Discharge System of shiny salts. At the conclusion of the cycle, the vacuum is unveiled along with the contents in the boiler are eliminated manually which has a scraper blade as a result of on the list of front doorways. Crystallizers can get the job done properly with warmth pumps, steam, or hot h2o. This strategy is Just about the most common ways of reaching increased industrial efficiency and this method also is determined by the properties and features.
can be utilized by yourself or at the side of other systems similar to a brine concentrator or an evaporator. Steam-pushed evaporators evaporate water from an answer or slurry, although the output is still liquid as an alternative to crystal. An item is concentrated all through evaporation by boiling the solvent, which is normally drinking water.
6, and that is in keeping with that on the raw concentrated SWRO brine (thirteen.one), suggesting the magnesium would not selectively accumulate during the internal facet nor the floor side. This element is one more reason for the excellent extensive-expression Procedure security from the 3D photo voltaic crystallizer as it ensures a secure porous construction of the salts crust layer. All of these success strongly assistance the success of our proposed method of salt crystallization inhibition to manage pore spaces of crystallized salt aggregates.
In this method, the solutes have to have a melting curve that will be able to minimize in temperature If your crystallization method utilizes supersaturation by cooling. The whole process of supersaturation is reached by evaporation of solvent only exactly where the solubility is dependent upon the temperature.